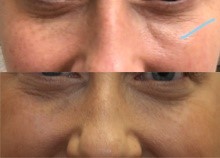
In my medical practice, I dissolve filler with an enzyme called hyaluronidase daily. The vast majority of these are for people who’ve had filler injected elsewhere. Why do I see so many people who need this? For several reasons. There are so many providers that perform injections with different skill sets and different backgrounds. These include nurse injectors, doctors who dabble in fillers as a “side job” to their primary medical specialty, and injectors who don’t have as much insight into the filler types and techniques required around the eyes as an oculoplastic surgeon does.
In this post I describe which fillers can be dissolved, how the filler dissolving enzyme works, and what data is available in the literature to speak to the safety of this procedure. At the end I provide a list of references that represent an overview of the current medical literature on off-label hyaluronidase for cosmetic fillers.
There are 3 main reasons people have filler dissolved:
Which fillers are reversible?
The magic of hyaluronic acid (HA) gel fillers are that they can be dissolved with the injection of an enzyme called hyaluronidase. So if you don’t like your HA filler you’re not necessarily stuck with it.
There are non-dissolvable fillers on the market like hydroxylapatite (Radiesse), poly-l-lactic acid (Sculptra), polymethyl methacrylate (Bellafill), and off-label liquid silicone (Silkon-1000) which are not reversible or dissolvable with hyaluronidase. Permanent, semipermanent and biostimulatory fillers are not used as commonly as HA fillers.
What exactly is a hyaluronic acid filler?
Hyaluronic acid is a substance that occurs naturally in the body (skin, soft tissue, joints, eye) with a half-life of approximately 24 hours and consists of a chain of sugar molecules (repeating disaccharides D-glucuronic acid and N-acetyl-D-glycosamine) called a polysaccharide. In the skin, HA is synthesized by dermal fibroblasts and epidermal keratinocytes. In cosmetic fillers, the chains can consist of different lengths and in turn, weights. The longer chains are considered high-molecular-weight and the shorter are low-molecular-weight. Some fillers have one or the other or a mixture of these. These strings or chains of molecules are held together with weak hydrogen bonds, keeping the disaccharides together but also linking the chains to themselves and each other. The chains on their own tend to disperse rather quickly (days to weeks) and some of these free chains are found in all dermal fillers, but manufacturers came up with a way to make the fillers last longer.
Commercially available hyaluronic acid gel fillers are manufactured in a medical facility via streptococcus biofermentation and are made to last longer by the addition of stronger bonds between the molecules. These bonds are referred to as “cross-linking” and can consist of divinyl sulfone or butanediol diglycidyl ether (BDDE) for example. The length of the polysaccharide chains (particle size) and the types of chain mixtures and cross-linking and the overall concentration of HA contribute to the thickness of the filler and the way the gel behaves under the skin. It should be noted that the chemical compounds used to cross-link the fillers are a potential source of allergy reaction for some individuals, some compounds more so than others.
The term rheology refers to the properties of the filler and doctors choose fillers for different applications based on these properties. The stiffness of the filler or G’, the cohesivity or how it holds it’s shape, the viscosity or how thick it is, and the water binding capacity or how much it causes swelling to occur after injection are all influenced by the constitution of the filler.
Hyaluronidase – the enzyme that dissolves filler
In the body, both the natural hyaluronic acid and the hyaluronic acid injected in the form of fillers will degrade over time by reactive oxidative species (free radicals) and by an enzyme produced in the body called hyaluronidase. This enzyme, part of a group called endoglycosidases will break apart the chains of polysaccharides by hydrolysis at specifically targeted links in the chain (the glucosaminidic linkage between C1 of the N-acetylglucosamine and C4 or glucuronic acid). By breaking the longer chains into shorter segments, the hyaluronic acid becomes more liquid and loses it’s form. The body then clears the components of the chains, primarily through the liver.
Hyaluronidase works primarily on hyaluronic acid but to a lesser degree on other mucopolysaccharides in connective tissue such as glycosaminoglycans in the skin’s extracellular matrix. It does not affect fibroblast activity or collagen in the skin itself (collagenase is an enzyme that degrades collagen).
Hyaluronidase has been used since the late 1940’s in medicine. It’s official FDA-approval is for the dispersion of injected medications, to combat dehydration, and to help with the visualization of contrast dyes in certain imaging studies. These indications work because of the enzyme’s action on the extracellular matrix. It’s often used off-label to help spread an injected anesthetic (as in periocular injections for ophthalmic surgery), to lyse epidural adhesions in the setting of back pain, to reduce edema, and to dissolve cosmetic fillers.
The different forms of hyaluronidase
This enzyme been manufactured in several forms, originally derived from cow (Amphadase, Hydase, and compounded forms) and sheep (Vitrase), and currently as a human recombinant form (Hylenex).
The animal-derived hyaluronidases have been losing favor due to variability in potency, purity, and the potential for IgE-mediated allergic reactions to factors found in the formulations. The human recombinant hyaluronidase (rHuPH20 or Hylenex) is manufactured in genetically engineered Chinese Hamster Ovary cells with a DNA plasmid insertion that encodes for a soluble fragment of human hyaluronidase. It has an improved safety profile than the animal-derived products likely due to it’s improved purity and was approved by the FDA in 2005.
It’s recommended that hyaluronidase is stored at cool temperatures to maintain product activity.
Researchers have developed recombinant bacterial hyaluronidases but these are not commercially available at this time.
How long it takes to dissolve filler
The half-life of hyaluronidase differs in different tissues but can range from 1 day to 4 days, so some effect is seen instantly when dissolving filler, but this effect continues for at least a day or more. The effect depends on how much enzyme is injected, how close the enzyme is to the filler, how the filler is distributed, how much filler is present, and what type of filler is being targeted.